Rebuilding high-stakes software AI-first: Delfa’s Participant Relationship Management System
Why AI-first systems are emerging first where failure is not an option.
The need for a clinical trial operating system
Every industry has its high‑stakes systems: software that cannot fail because the cost of failure is borne by real people. In clinical research, that system is the infrastructure that manages participant journeys through clinical trials. These trials are the gateway through which every new therapy must pass, and their performance determines how quickly medicines reach patients.
Yet this critical layer has long been held together by inboxes, spreadsheets, and heroic vigilance. Trials generate a constant stream of emails, phone calls, documents, reschedules, protocol changes, and participant questions. In fact, trials now generate >250% more data points compared to 10 years ago, leading teams to compensate with manual effort and constant oversight. The outcome is predictable: delays, deviations, and participants waiting longer than they should for treatments that could change or save their lives.
This pressure is only increasing. Over the past decade, AI-first biotech companies have expanded the number of viable drug candidates reaching the clinic. Indeed, the second generation (founded ‘18-’21) of AI biotechs alone is expected to bring more drugs into the clinic in 2026 than the first generation (founded ‘11-’14) managed across multiple prior years. The bottleneck has shifted from discovering molecules to running trials fast enough, cleanly enough, and at sufficient scale to keep up.
That’s why earlier this year we invested in Delfa. The company is rebuilding one of the most critical pieces of clinical trial infrastructure from first principles. With its Participant Relationship Management (PRM) system, Delfa is taking the next step toward rewriting the operating system for clinical trials.
Managing participant relationships at scale
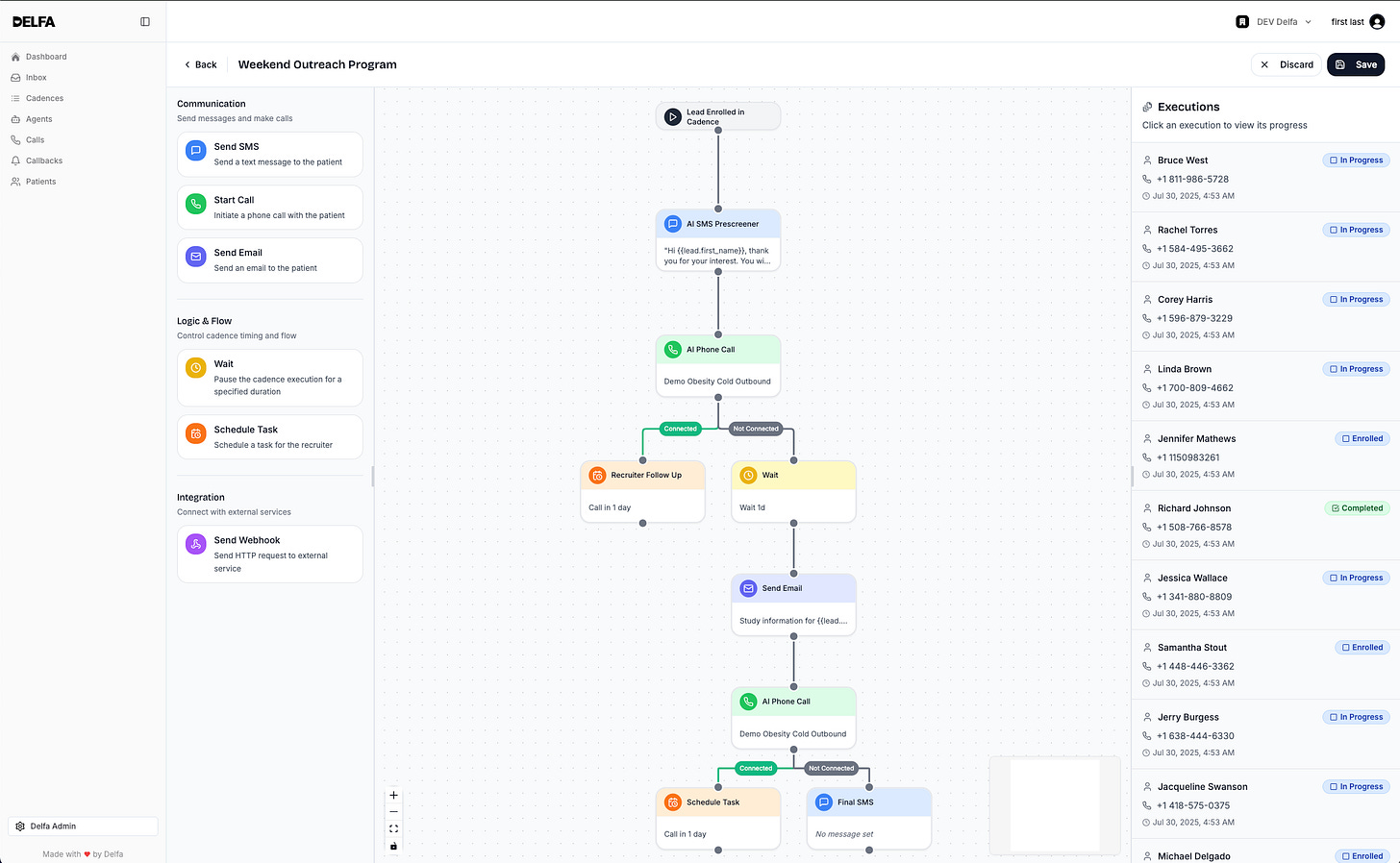
The Delfa PRM is built on modern AI systems that can interpret unstructured communication, understand documents, coordinate tasks, and maintain context across an entire participant journey. Instead of acting as a passive system of record, it operates continuously in the background. The system does the work, rather than asking humans to do it on its behalf.
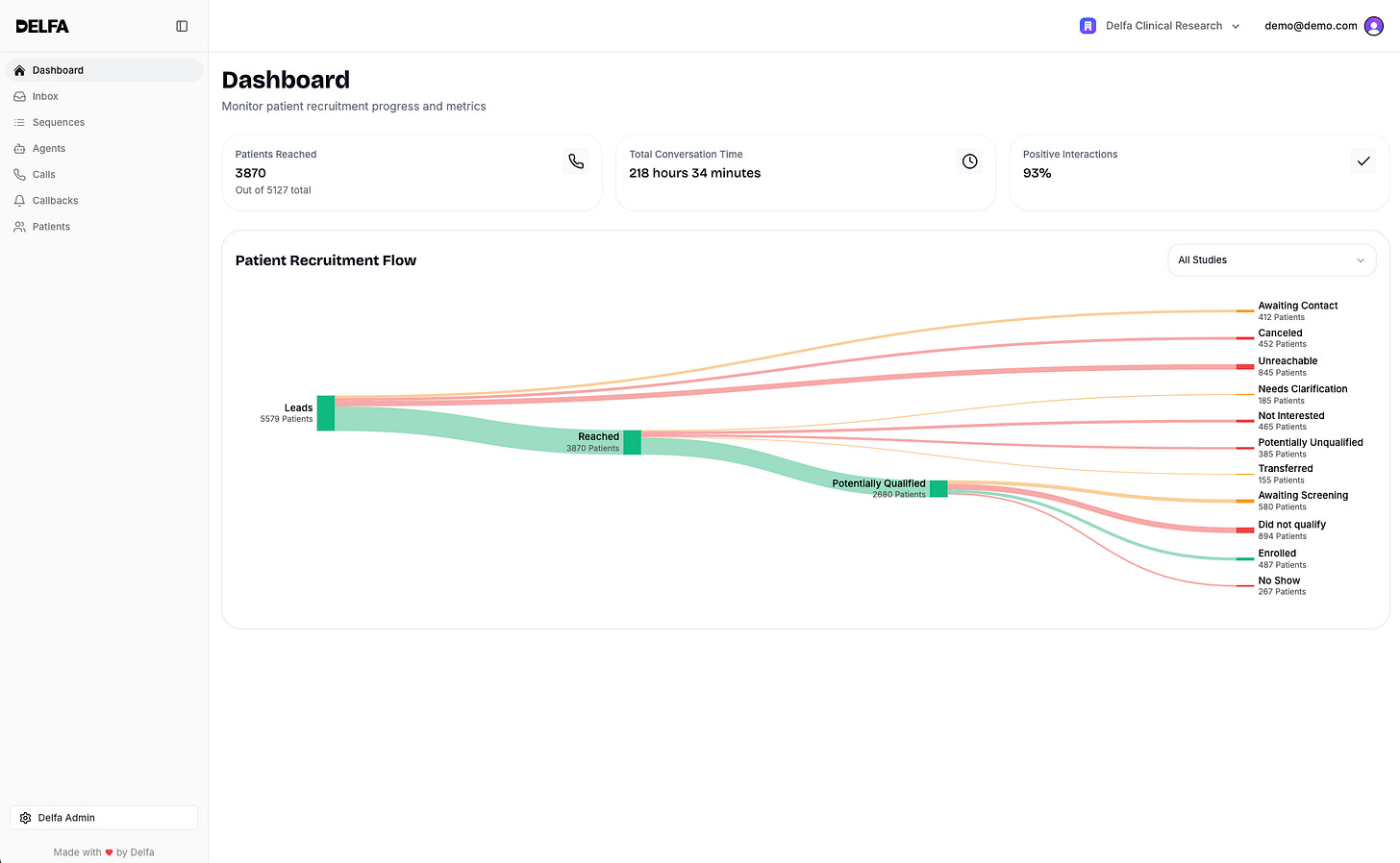
With Delfa PRM, clinical trial sites get:
A unified inbox where AI triages communication and extracts structured data.
Protocol-aware workflows that generate and manage tasks automatically.
Scheduling that adapts to visit windows, constraints, and participant availability.
Real-time document understanding across forms, labs, and reports.
A complete operational memory of every interaction, without manual upkeep.
And automated, multi-channel text or audio-based recruitment sequences.
Taken together the impact is immediate for research sites: faster recruitment, fewer deviations, higher‑quality data, and staff freed from the coordination burden that has historically limited scale.
For participants, the impact is simpler and more important. Communication is consistent. Follow‑ups happen. Appointments stay on track. The experience feels human, not bureaucratic.
High-stakes software, AI-first
Delfa’s PRM also points to a broader shift underway across high‑stakes software categories. For years, enterprise software focused on digitising workflows and making work visible. As AI systems become capable of executing multi‑step, high‑variance tasks, the challenge has changed. The new constraint is no longer recording work, but orchestrating it reliably under real‑world conditions.
Delfa is doing exactly that for clinical research. PRM adds the operational layer that allows trials to run with the speed and precision the work demands. When trials run better, medicines move faster, development costs fall, and patients see the benefits sooner.
This is what rebuilding high‑stakes software AI‑first makes possible.




This resonates. What I’m tracking is the same shift you’re pointing to: from “AI as a tool” to AI as an operational layer—the part that makes high-stakes workflows actually run reliably in the real world. In that sense, PRM sounds less like automation and more like a system-level consistency mechanism: fewer deviations, tighter follow-ups, and an experience that feels human (not bureaucratic).
I explored a related idea as a “meta-layer” that helps AI notice drift, compare against standards, and correct itself iteratively—so the process improves, not just a single output. If that framing is useful, here’s the piece: https://northstarai.substack.com/p/ai-spoke-of-a-meta-layer-in-its-own
Curious: where do you see the biggest failure mode—handoffs, compliance, or long-horizon coordination?